Inhibitory Proteins Are Encoded By
The jail cell wheel is the series of events which regulate the life of the cell. This regulation results from a combination of several signals from unlike regulatory pathways that are activated in response to specific stimuli. The cell wheel has a central role in controlling cell growth and proliferation. It frequently becomes the target of genetic alteration, the accumulation of which may lead to the deregulation of these ordered events and may be related to the onset of cancer. With the growing understanding of the of import role of prison cell bicycle regulation in tumor formation and apoptosis, jail cell wheel inhibitors have been further studied in the field of cancer handling.
Cyclin-dependent Kinase (CDK) Inhibitors
Progression through the cell cycle is ensured by particular protein complexes, the cyclin-dependent kinases (CDKs). The CDKs are a family of highly conserved serine/threonine kinases, which share a high homology in a detail region. CDKs may control the cell cycle by phosphorylating different targets, which may in plough be activated or inactivated. Regulation of CDK/cyclin activity can occur through regulatory proteins, such equally CDK Inhibitors (CKIs). The CKIs can inhibit the action of CDK past associating in vivo with the CDK subunit, the cyclin or the cyclin/CDK complex. This inhibition may occur in unlike ways, such every bit inhibition of the CDK kinase activity, interference with CAK mediated CDK activation, or competition with cyclins in binding to the catalytic subunit. The inhibitory procedure tin can be carried out by ane or a combination of these mechanisms. The expression of these CKIs may be induced by stimuli such every bit senescence, contact inhibition, extracellular anti-mitogenic factors and cell cycle checkpoints.
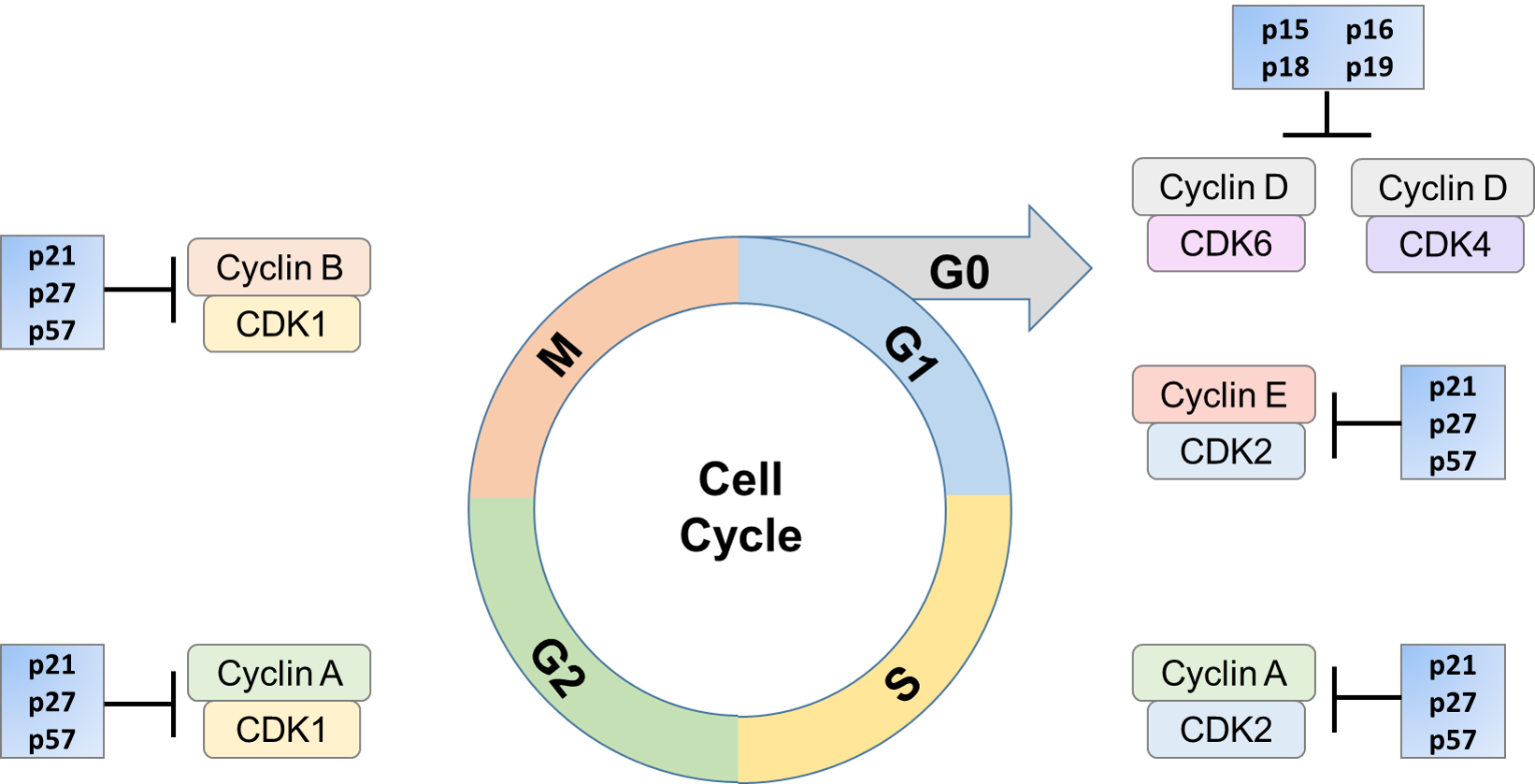
Their role in controlling prison cell bike is crucial. In several forms of cancer, CKIs such equally p16 and p27 are mutated. Also, they have been found to exist degraded in several types of cancer. Low levels of p27 levels are correlated with poor clinical prognosis. These inhibitors tin be upregulated when required, thus blocking the activation of the CDK by a cyclin. This arrests the cell in a detail office of the cell bike until conditions are such that it can keep towards proliferation or, if necessary, exist steered towards cell expiry.
- INK4 Family
There are two families of CKIs. The starting time family includes the INK4 proteins (inhibitors of CDK4), so named for their power to specifically inhibit the catalytic subunits of CDK4 and CDK6. This family includes four proteins, p16INK4a, p15INK4b, p18INK4c, and p19INK4d. These proteins are composed of multiple ankirin repeats and demark only to CDK4 and CDK6.
| INK4 | Clarification |
| p15 | Cyclin-dependent kinase 4 inhibitor B, too known equally multiple tumor suppressor 2 (MTS-2) or p15INK4b, is induced by TGF treatment and is expressed ubiquitously. The protein is encoded by the CDKN2B gene in humans, which is frequently deleted in human cancers, suggesting that the loss of the gene may exist significant in the development of certain types of tumors. |
| p16 | p16, also known as p16INK4a, cyclin-dependent kinase inhibitor 2A, and multiple tumor suppressor 1, is encoded by the CDKN2A gene in humans. Information technology was identified in a ii-hybrid screening using CDK4 every bit a bait. p16 tin block CDK4 and CDK6 function past sequestering the catalytic subunit, or by blocking the kinase activity of preassembled complexes. Due to the fact that several human cancers are associated with loss of p16 function, this gene was proposed as a tumor suppressor in vivo. |
| p18 | p18, too known as cyclin-dependent kinase 4 inhibitor C, is an enzyme that in humans is encoded past the CDKN2C factor. p18 has been shown to collaborate with CDK4 or CDK6, and forestall the activation of the CDK kinases, thus office equally a cell growth regulator that controls cell cycle G1 progression. Both p18 and p19 are widely distributed in different cell types and tissues. |
| p19 | p19, also known as cyclin-dependent kinase 4 inhibitor D, is an enzyme that in humans is encoded by the CDKN2D gene. In some cell types, p19 levels oscillate during the jail cell bicycle, undergoing induction when cells enter S stage. p19 may function past regulating the activity of cyclin D-dependent kinases as cells leave G1 phase. All four inhibitors share similar properties and may respond differently to anti-proliferative signals. |
- CIP/KIP Family
The latter family is composed of the members of the Cip/Kip family, and includes p21Cip1, p27Kip1 and p57Kip2, all of which contain characteristic motifs within their amino-terminal moieties that enable them to bind to both cyclin and CDK subunits. Members of the Cip family bind to and inhibit the agile cyclin/CDK circuitous.
| CIP/KIP | Clarification |
| p21 | p21Cip1, likewise known every bit cyclin-dependent kinase inhibitor 1 or CDK-interacting poly peptide one, is encoded by the CDKN1A cistron in humans, and can act as a stiff and universal inhibitor of CDK activeness. Information technology inhibits CDK2, CDK4, and CDK6 kinases and is capable of inducing cell cycle arrest in G1 when overexpressed. In normally cycling cells, p21 is in complex with cyclin/CDK. |
| p27 | Cyclin-dependent kinase inhibitor 1B (p27Kip1) is an enzyme inhibitor that in humans is encoded by the CDKN1B cistron. p27 was identified in a series of studies on growth inhibitory activity induced past TGF, and in some other written report was isolated by a 2-hybrid screening, using CDK4 as a allurement. p27 is structurally related to p21 and inhibits CDK2, CDK3, CDK4, and CDK6 complexes in vitro. |
| p57 | Cyclin-dependent kinase inhibitor 1C (p57, Kip2) is poly peptide which in humans is encoded past the CDKN1C cistron. p57 binds to and inhibits several cyclin/CDK complexes and its expression seems to be tissue-restricted. p57, like p27, appears not to require p53 and pRb for its function. p57 maps to the 11p15 chromosomal locus, which undergoes frequent deletions or rearrangements in many forms of human cancer. |
Tumor Suppressor and Prison cell Cycle Command
Tumor transformation is a multi-footstep process involving the clonal aggregating of genetic lesions affecting proto-oncogenes or tumor suppressor genes. The products of these genes later on play an important role in signal transduction pathways by controlling cell cycle, cell differentiation and even jail cell decease. There is now increasing prove that normal cell cycle progression is the consequence of a balanced interaction between multiple regulatory factors, such as tumor suppressor gene products and jail cell cycle-associated proteins. It is not surprising that basic changes in tumor suppressor genes may result in unregulated cell cycle and eventual tumor transformation. Therefore, people may define cancer as a genetic illness of the cell cycle.
The prototype tumor-suppressor genes are p53 and RB. The involvement of these tumor-suppressor genes in cancer is widespread and often the genes show smashing specificity for particular tumor types. The most mutual amending institute in these genes is represented by deletion or bespeak mutation. In addition, inactivation of tumor-suppressor genes may result from interaction with viral oncoproteins. In fact, viral agents are capable of integration into the host's genetic material and may interfere with the regulation of normal prison cell growth and proliferation by interacting with the function of tumor suppressor genes such as the p53 and pRb families.
Cell Regulation and Cell Cycle Control
The cell bicycle is not an contained cellular machinery, but a process that is closely related to other cellular regulatory mechanisms. For example, proteins that regulate chromatin remodeling also affect the cell cycle, such as the ADNP gene; proteins that inhibit cellular energy metabolism besides inhibit the cell cycle, such as ALDH1L1; proteins that inhibit cancer cell growth and chromatin condensation can also act equally cell wheel inhibitor proteins, such as ASB2. Other jail cell procedure, including apoptosis, cell growth, ubiquitination, calcium responsive transcription, et al., can affect the normal prison cell cycle. Cell biological science is a complex and interacting network, and then to fully understand the mechanisms of prison cell wheel inhibitors, you need to empathize the multiplex cell regulation procedure.
Determination
Jail cell cycle inhibitors have broad prospects in the field of human cancer therapy. A diversity of CDK inhibitors are in the clinical and preclinical trials. Previous studies have demonstrated that these drugs tin can inhibit the jail cell bike and induce apoptosis in tumor cells. In clinical trials, the all-time effect these drugs tin reach is to stabilize the status. In full general, cell bicycle inhibitors are ane of the constructive strategies for cancer treatment and are worthy of in-depth experiments and research.
Reference:
ane. Antonio Giordano Chiliad D, Soprano G J. Cell Cycle Inhibitors in Cancer Therapy [M]. Humana Printing, 2003.
Inhibitory Proteins Are Encoded By,
Source: https://www.creativebiomart.net/researcharea-cell-cycle-inhibitor-proteins_404.htm
Posted by: smithtient1979.blogspot.com


0 Response to "Inhibitory Proteins Are Encoded By"
Post a Comment